A simulation-based imaging study found that in patients with severe asthma, airway structure, and type-2 inflammation may influence the delivery of inhaled corticosteroids. Extrafine-particle formulations showed better overall deposition than fine-particle steroids but did not fully overcome delivery challenges in patients with altered airway morphology.
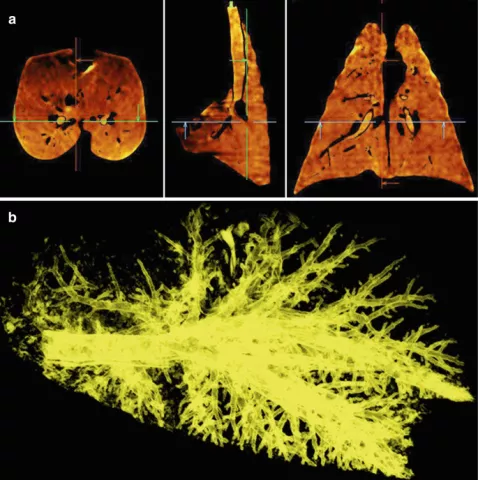
This study aimed to compare the model-predicted deposition of fine-particle inhaled corticosteroid (ICSFP; fluticasone-propionate HFA) and extra fine-particle corticosteroid (ICSEFP; beclomethasone-dipropionate HFA) about airway structure, function, and type-2 inflammatory biomarkers. Twenty-eight patients with severe asthma underwent full-inspiration and full-expiration chest CT scans on the same day type-2 inflammatory markers were assessed. Functional respiratory imaging and computational fluid dynamics were used to simulate intrathoracic, central, and peripheral airway deposition, along with central-to-peripheral (C:P) deposition ratios. Airway morphology was measured using CT-derived wall area percent (WA%), lumen area (LA), and mucus burden.
Simulated deposition of ICSEFP was significantly higher than ICSFP in all airway regions (intrathoracic, central, and peripheral; all p<0.0001). A greater WA% and smaller LA were associated with higher C:P ratios, suggesting reduced peripheral deposition. This was observed for both ICSFP (WA%: r=0.60, p=0.0068; LA: r=−0.60, p=0.0072) and ICSEFP (WA%: r=0.54, p=0.028; LA: r=−0.54, p=0.026). Participants with elevated sputum eosinophils showed significantly higher C:P ratios, regardless of particle size (ICSFP: p=0.045; ICSEFP: p=0.021), indicating inflammation-related barriers to steroid delivery.
Airway remodeling and inflammation may limit corticosteroid delivery to peripheral airways. Extrafine particles improved deposition but did not fully overcome this barrier. Despite high-dose therapy, patient-specific airway structure and inflammation may contribute to persistent type-2 inflammation.